In Silico Medical & Biomedical Device Testing: Finite Element, CFD & Electromagnetic Simulation and Design, Considering FDA & ASME V&V 40
FEA & CFD Based Simulation Design Analysis Virtual prototyping MultiObjective Optimization
FEA and CFD based Simulation design and analysis is playing an increasingly significant role in the development of medical devices, saving development costs by optimizing device performance and reliability, reducing benchtop tests and clinical trials, and helping to speed the regulatory approval process. Developing increasingly complex medical devices requires increasingly capable tools for simulation and testing. Our deep portfolio of simulation and testing solutions allows medical device developers to generate digital evidence of device performance across a range of engineering disciplines, throughout the product life cycle.
Enteknograte offers a Virtual Engineering approach with CFD and FEA tools such as Ansys Fluent and Siemens StarCCM+ for flows simulation and FEA Codes such as Abaqus, Ansys, Nastran and LS-Dyna, encompassing the accurate prediction of in-service loads, the performance evaluation, and the integrity assessment including the influence of manufacturing the components for In Silico Medical & Biomedical Device Testing with Finite Element & CFD Simulation, Considering FDA & ASME.
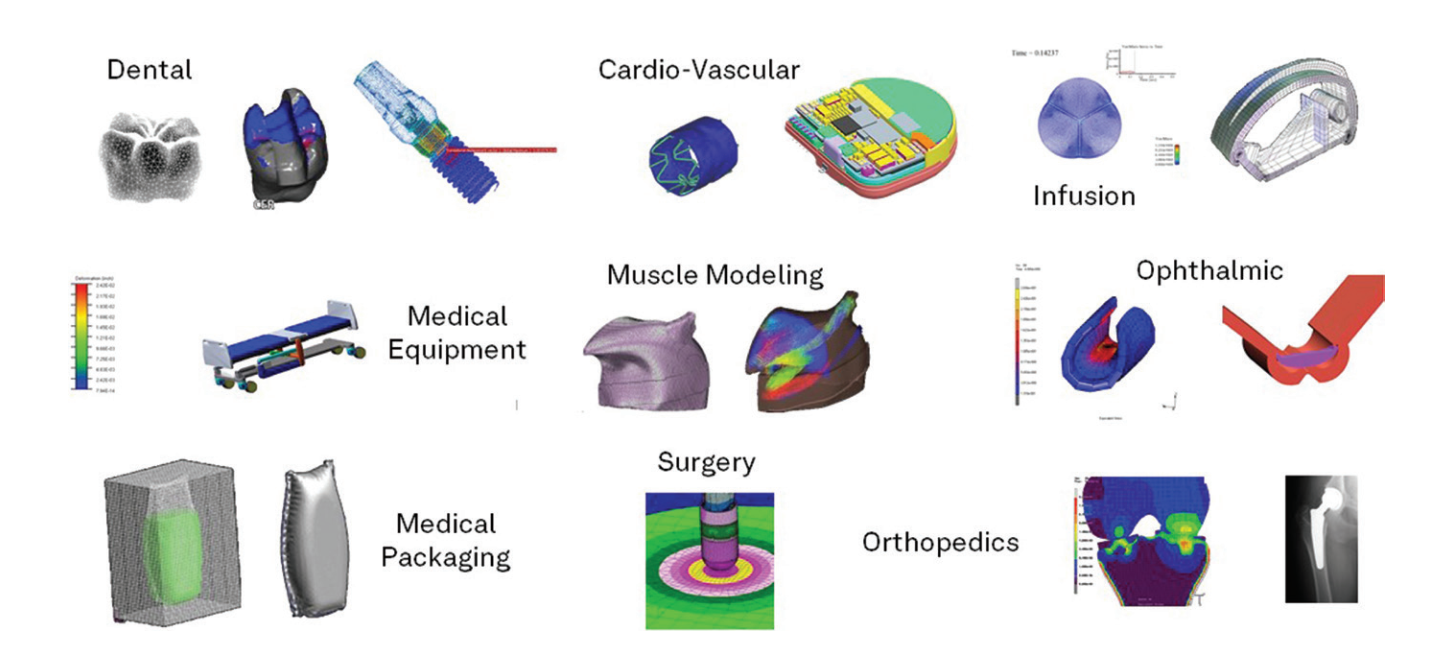
Enteknograte Biomedical Engineers use advanced FEA and CFD tools for simulating: Orthopedic products, Medical fasteners, Ocular modeling, Soft tissue simulation, Packaging, Electronic systems, Virtual biomechanics, Knee replacement, Human modeling, Soft tissue and joint modeling, Hospital equipment, Laser bonding, Ablation catheters, Dental implants, Mechanical connectors, Prosthetics, Pacemakers, Vascular implants, Defibrillators, Heart valve replacements.
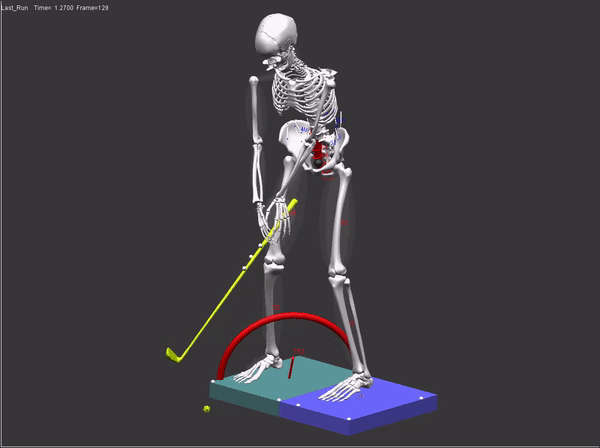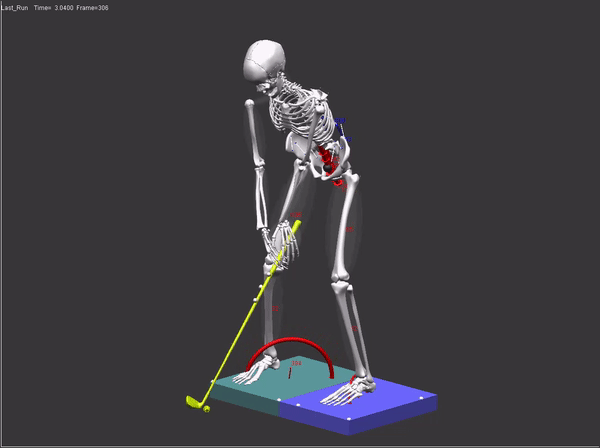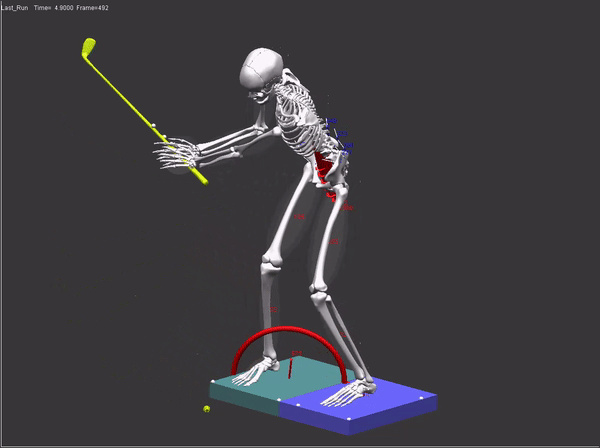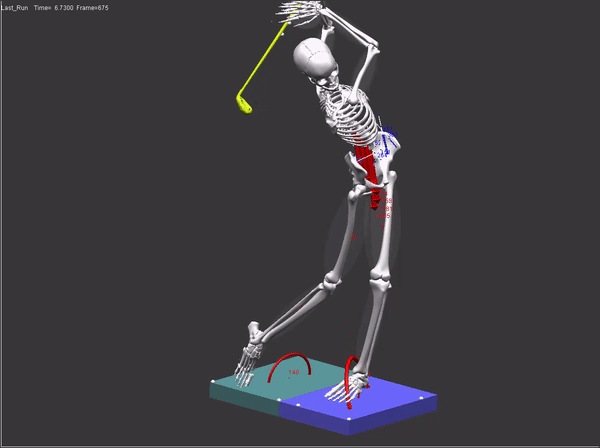
FDA submissions require a comprehensive audit trail which includes:
- Records of all simulation data
- Revision management and control
- Correlation with physical test results
- Proven repeatable methods
- Software version tracking
To ensure confidence in the simulation and reliability of the analysis results, medical device manufacturers must ensure that the CAE analyst is using the most current CAD geometry, specify the correct material properties, and apply the appropriate environmental loading conditions. They must also create the proper mesh type and size, record the version of the analysis software, and correlate results with physical test data. These criteria must be preserved and managed for FDA compliance purposes and for the benefit of enhanced enterprise productivity.
FDA Describes the Format for Reports on Computational Modeling and Simulation
This guidance’s main objective is to make sure sponsors provide all the details necessary to assess if a model has the necessary credibility to be used as reliable scientific evidence in regulatory decision-making. Additionally, the document offers a consistent vocabulary that regulators and the device industry can use when organizing, debating, and evaluating the various components of a computational modeling research.
The majority of the guidelines, which is applicable to the majority of physics disciplines, describes the report’s overall format. The guidance also includes a number of appendices that offer comprehensive reporting recommendations for CFD, FEA, electromagnetics (EM), ultrasound, heat transfer, and optics in order to help the user. For instance, the appendix on computational electromagnetics advises the user to provide a synopsis of the governing equations used (for instance, Maxwell’s equations) and details on the electrical characteristics of the device and tissues being represented.
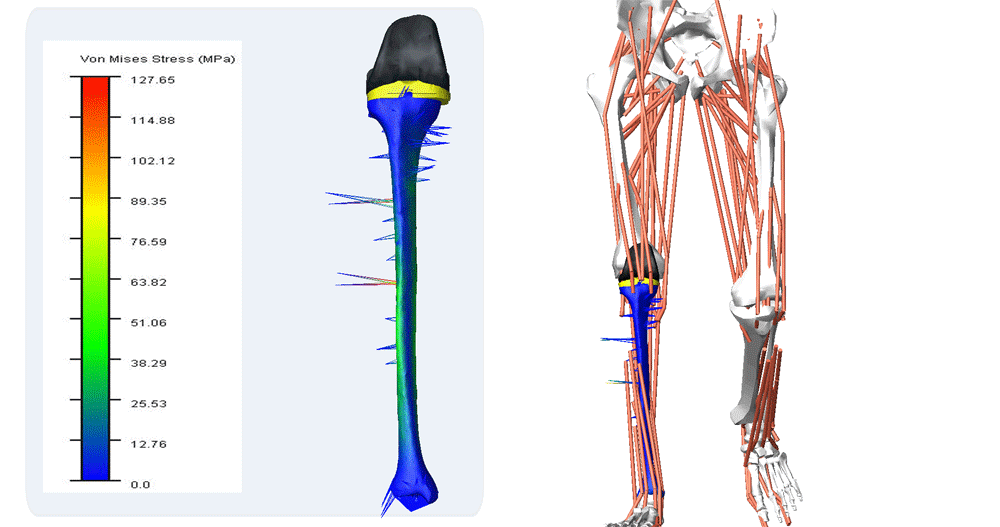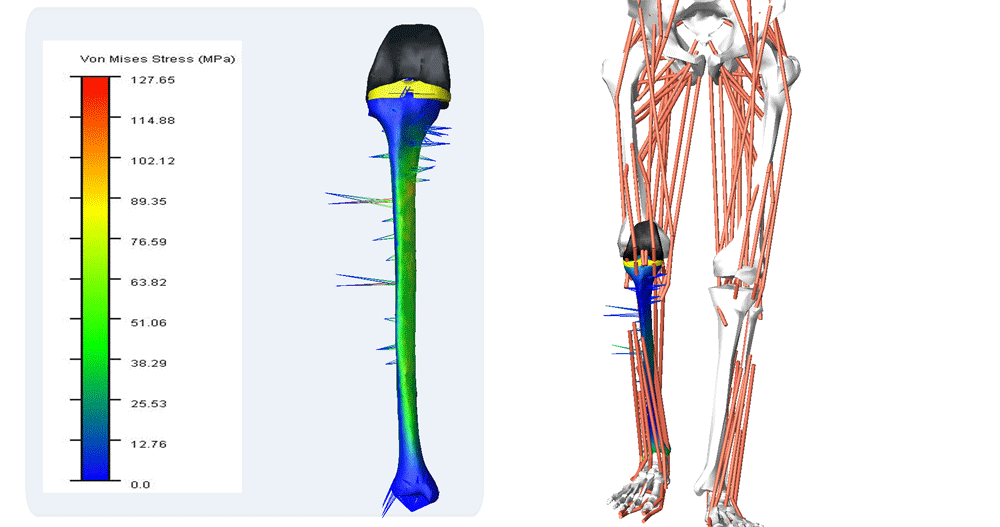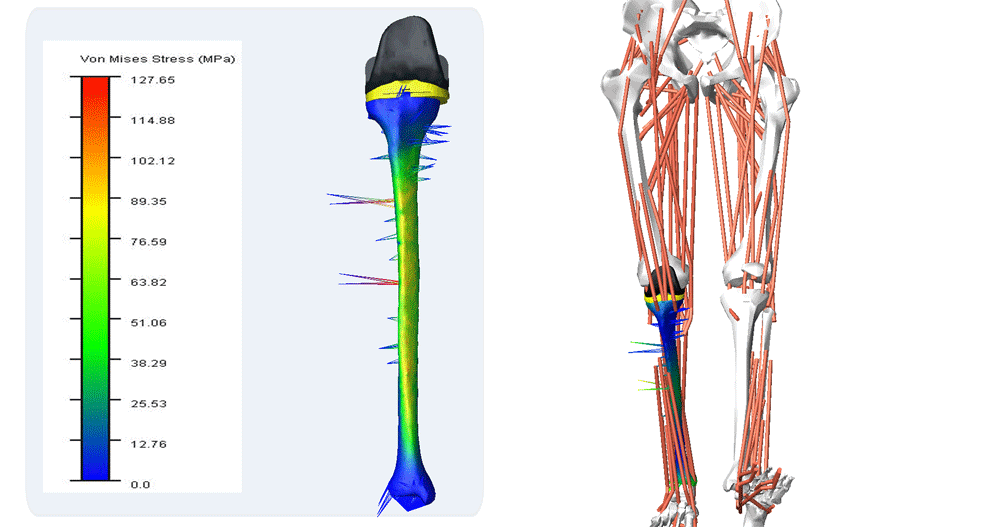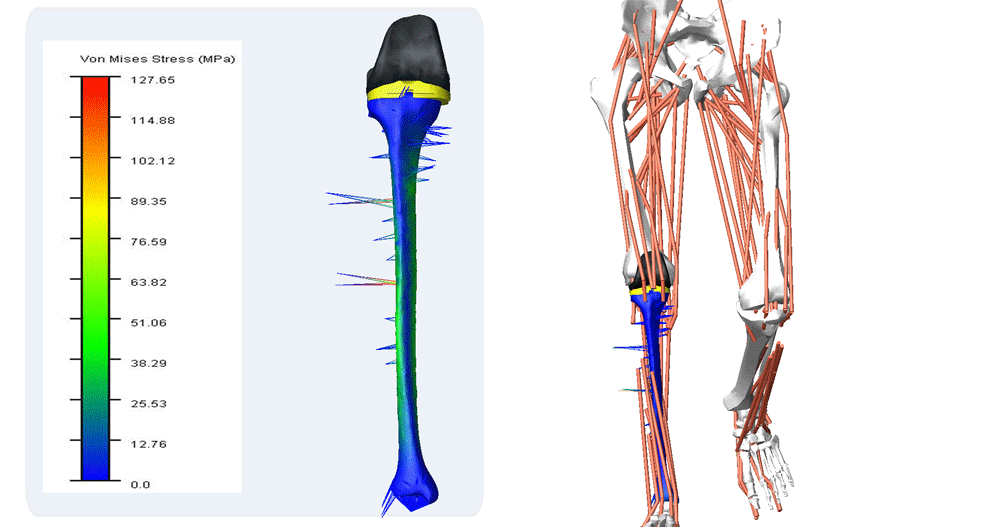
Reporting of Computational Modeling Studies in Medical Device Submissions, Guidance for Industry and Food and Drug Administration Staff
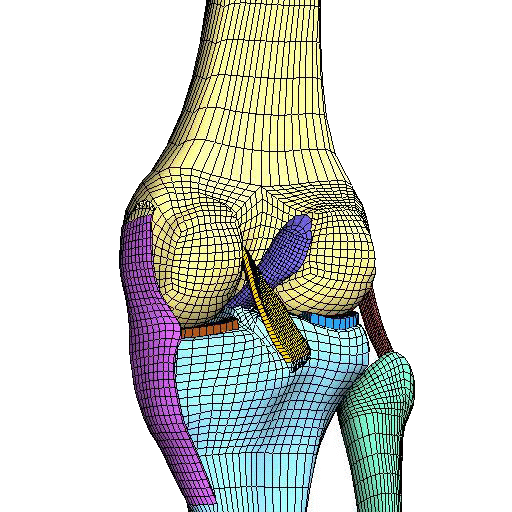
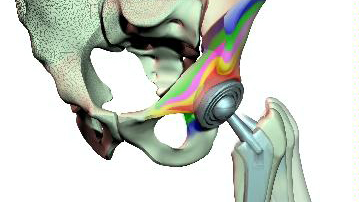
For many years, computational modeling and simulation (CM&S) studies have been used by sponsors to support device design/development and have been reported in medical device submissions. These studies have traditionally been used in the areas of fluid dynamics (e.g., calculate shear stress in ventricular assist devices), solid mechanics (e.g., determine maximum stress locations in a hip implant), electromagnetics and optics (e.g., radiofrequency safety in magnetic resonance imaging, fluorescence for fiber optic spectroscopy devices), ultrasound propagation (e.g., absorbed energy distribution for therapeutic ultrasound), and thermal propagation (e.g., temperature rises with radiofrequency and laser ablation devices).
The purpose of this guidance document is to provide recommendations to industry on the formatting, organization, and content of reports of CM&S studies that are used to support medical device submissions. Moreover, this guidance is also for FDA Staff, to improve the consistency and predictability of the review of CM&S studies and to better facilitate full interpretation and complete review of those studies. The FDA guidance used in conjunction with ASME V&V 40. The ASME V&V 40 standard is an FDA-recognized standard that provides a risk-based framework for establishing the credibility requirements of a computational model.
The risk-informed credibility assessment framework, as defined by the ASME V&V 40 standard for In Silico Medical Device Testing
Another reason for the limited utilization of simulation results in regulatory submissions was the need for consensus on the evidentiary bar required to establish sufficient credibility of a computational model. The American Society of Mechanical Engineers (ASME) verification and validation (V&V) subcommittee on computational models of medical devices (ASME V&V 40 subcommittee) was formed in 2011 to address this critical need.
Comprised of members from the medical device industry, industry service providers, software developers and the FDA, the group developed the risk-informed credibility assessment framework. The main tenet of the framework is that high-risk decisions based on a computer model require more validation than lower-risk decisions.
Therefore, the ASME standard guides how the user determines the amount of V&V that is required to establish model credibility. This standard complements the ASME V&V standards for FEA, CFD and heat transfer.
- Risk-informed credibility assessment framework.
- Finite element Simulation framework, as defined by the ASME V&V 40 standard for In Silico Medical Device Testing
- CFD and Thermal Simulation framework, as defined by the ASME V&V 40 standard for In Silico Medical Device Testing
- VVUQ 40 VERIFICATION, VALIDATION, AND UNCERTAINTY QUANTIFICATION IN COMPUTATIONAL MODELING OF MEDICAL DEVICES
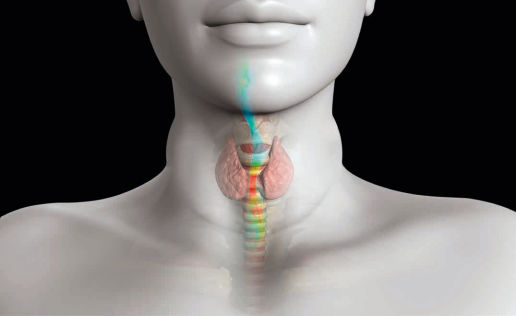
Medical Device Electromagnetic Performance and Safety
Advanced electromagnetic simulation technology has indeed revolutionized the field of medical device development and validation. By leveraging computational modeling tools, device manufacturers can assess and validate the electromagnetic performance, safety, and compliance of their products in a virtual environment.
Some key applications and benefits of advanced electromagnetic simulation in the context of medical devices:
- Antenna Design and Placement: Simulation tools enable the design and optimization of antennas used in medical devices such as wireless communication systems, implantable devices, and wearables. Virtual validation allows for efficient exploration of various antenna designs and their optimal placement, improving device performance and reducing the need for physical prototyping.
- Signal and Power Integrity Assessment: Electromagnetic simulation aids in analyzing signal integrity issues, such as crosstalk, electromagnetic interference, and power distribution problems. By identifying and mitigating these issues early in the development process, manufacturers can optimize the functionality and reliability of their medical devices.
- EMI/EMC Evaluation: Electromagnetic Interference (EMI) and Electromagnetic Compatibility (EMC) are critical aspects of medical device safety and regulatory compliance. Advanced simulation tools can predict and evaluate EMI/EMC issues, allowing designers to identify potential interference sources and optimize device shielding, grounding, and filtering techniques.
- MRI Design: Magnetic Resonance Imaging (MRI) compatibility is crucial for devices used in proximity to MRI scanners. Electromagnetic simulation helps in assessing the behavior and potential hazards of medical devices in MRI environments. It enables designers to optimize device performance while ensuring patient safety and minimizing potential image artifacts or interference.
- Charged Particle Devices: Simulation tools can evaluate the behavior of charged particle devices, such as electron accelerators or proton therapy machines. By simulating the electromagnetic interactions and particle trajectories, manufacturers can optimize device design, improve treatment accuracy, and ensure patient safety.
- Human Exposure Compliance: Electromagnetic simulation can assess the exposure of virtual human models to electromagnetic fields generated by medical devices. This allows manufacturers to evaluate compliance with human exposure guidelines and optimize device designs to minimize potential risks
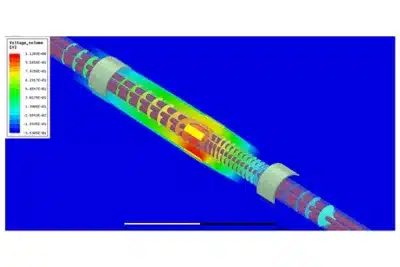
As part of our promise to excellency, we have experts with FDA's guidance for Reporting Computational Modeling and Simulation (CM&S) Studies in Medical Device Submissions.
Medical applications are typically subjected to a wide range of complex environmental and biological loading conditions.The variability of these conditions makes the physical testing of all possible scenarios both difficult and time-consuming. By using FEA and CFD multidisciplinary and multiphysics simulation technology, our engineers can study a greater number of real-world design behaviors with higher accuracy.
Finite element analysis of Orthopedic Implants
Finite element and CFD analysis and Digital Twin for Orthopedics, Dental and Medical Device Industry
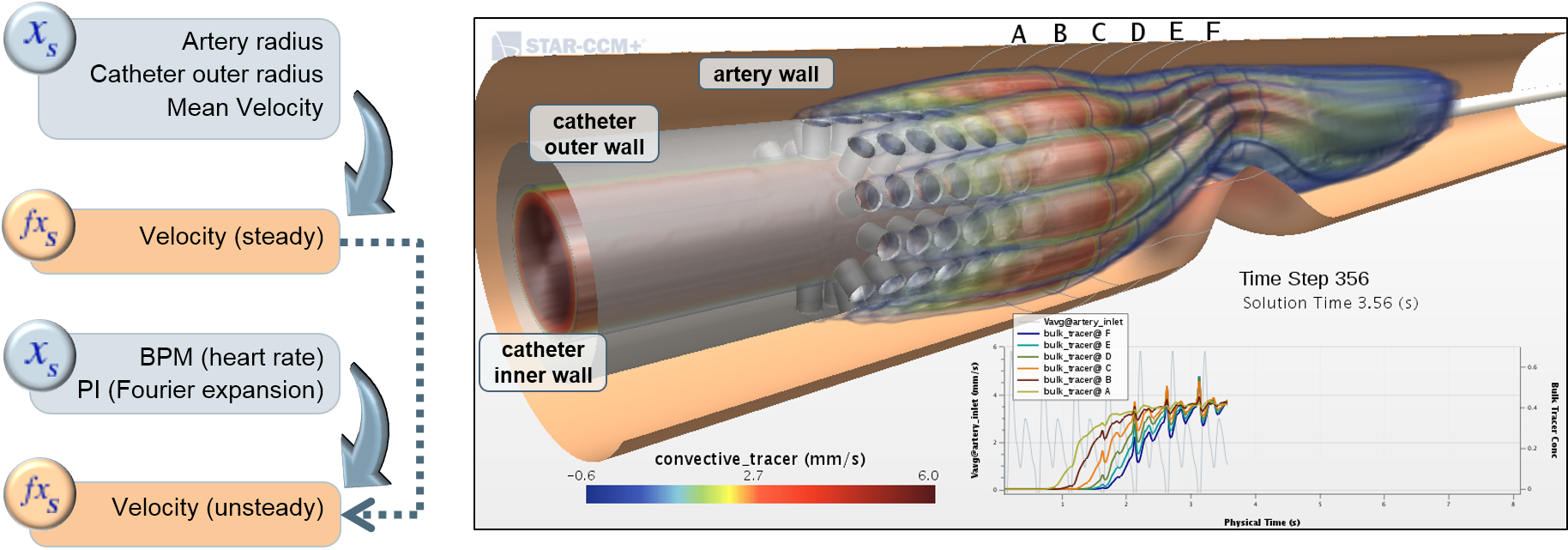
Finite element & CFD analysis of Cardiovascular Implants
Finite element analysis of Craniomaxillofacial and Plastic Surgery
Finite Element Analysis of Durability and Fatigue Life
Multi-Phase Flows CFD Analysis
Hydrodynamics CFD simulation
Integrated Artificial Intelligence (AI) & Machine Learning - Deep Learning with CFD & FEA Simulation
Enteknograte offers a Virtual Engineering approach with CFD and FEA tools such as Ansys Fluent, StarCCM+ for flows simulation and FEA based Codes such as ABAQUS, Ansys, Nastran and LS-Dyna, encompassing the accurate prediction of in-service loads, the performance evaluation, and the integrity assessment including the influence of manufacturing the components.
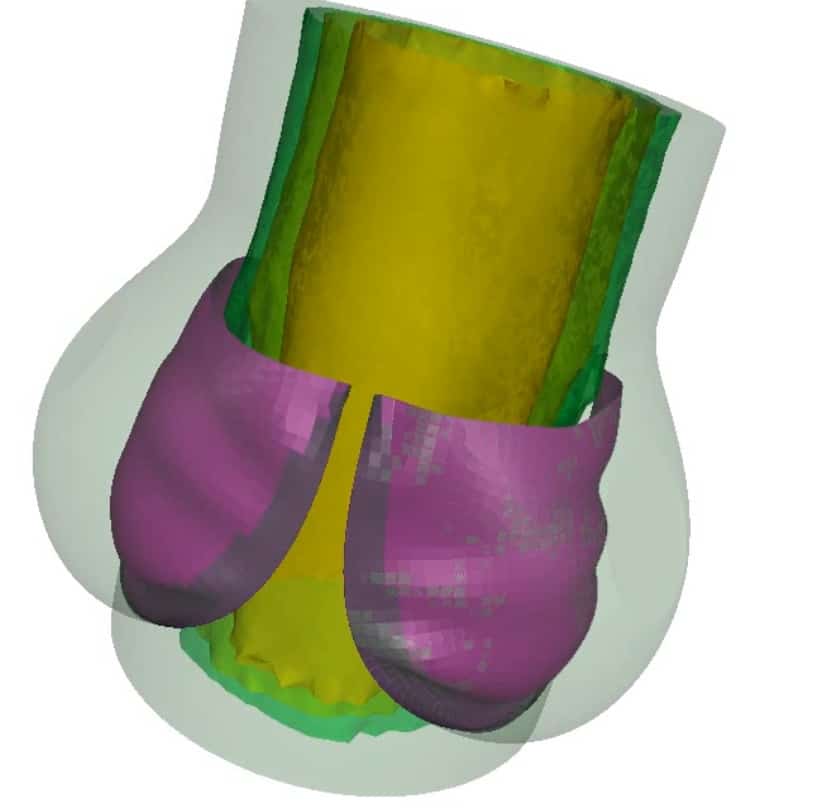
Multibody Dynamics
Electromagnetic Multiphysics
Ansys HFSS: Multipurpose High Frequency Electromagnetic Field Simulator for RF, Microwave and Wireless Design
Ansys Maxwell: Low-Frequency Electromagnetic Simulation for Electric Machines
Acoustics and Vibration: FEA and CFD for AeroAcoustics, VibroAcoustics and NVH Analysis
FEA Based Composite Material Design and Optimization: MSC Marc, Abaqus, Ansys, Digimat and LS-DYNA
Additive Manufacturing and 3D Printing
Heat Transfer and Thermal Analysis: Fluid-Structure Interaction with Coupled CFD and Finite Element Based Simulation




